MARC SE-AFRICA advancing data-led responses to drug-resistant malaria at ASTMH
Rising antimalarial resistance in Southern and East Africa risks reversing gains in malaria control. MARC SE-Africa assembles and analyses regional data on resistance to support fast, evidence-based policy.
On 11 November 2025, MARC SE-Africa and the Worldwide Antimalarial Resistance Network (WWARN) co-hosted a symposium at the American Society of Tropical Medicine and Hygiene’s annual meeting and presented some of the latest project milestones. Under the title “Antimalarial drug resistance in Africa: will we miss the train again?”, MARC SE Africa partners presented the latest data on the spread of antimalarial resistance markers, how patient outcomes after treatment align with the parasite mutations that are tracked for resistance, and how estimates can be made in cases where this data is sparse.
Individual patient data meta-analysis sheds light on resistance markers
MARC SE-Africa coordinator Prof Karen Barnes spoke about the findings from a large individual patient data meta-analysis that explored the relationship between mutations in the Kelch13 (K13) gene region of the Plasmodium falciparum malaria parasite and the growing resistance to artemisinin-based combination therapy (ACT).
Prof Karen Barnes presenting at the ASTMH.
This meta-analysis pooled data from 42 studies and approximately 14,000 individual patients, with over half of the studies (23) conducted in Africa. As the first analysis of its kind in scale and inclusiveness, it enhances our understanding of how K13 mutations relate to treatment response across Africa and Asia and supports the development of practical strategies against drug-resistant malaria on the African continent.
Several new insights have emerged from this work. Firstly, people clear malaria parasites at different rates depending on where they live, with slower clearance in settings with low malaria transmission intensity in Africa and much of Asia. Thus, the current global threshold for this rate will only account for one quarter (25%) of malaria infections in areas of moderate to high malaria transmission intensities.
Secondly, when the parasites carry these K13 mutations, infections clear more slowly and are more persistent after three days of treatment compared to the wildtype infections. More than one in 10 patients still having parasites present on Day 3 provides a simple alert that drug-resistant malaria may be established in that site. However, the accuracy of this method depends on first excluding patients with very high parasite loads (>100,000/uL) before treatment, who are more than twice as likely to have parasites persisting to Day 3, even when their infections are sensitive to first-line malaria treatment, artemisinin-based combination treatments (ACTs).
These findings confirm the urgent need to apply setting-specific alert thresholds for the rate of parasite clearance, and to routinely assess malaria parasite loads before treatment and three days after starting treatment. Wherever delayed clearance is common, an urgent review of the efficacy of the alternative ACTs in that area is needed for treatment policies to keep pace with changing parasite genetics.
The study group behind this work also recently published a protocol for a systematic review and individual patient data meta-analysis to serve as a model for further standardised statistical methodologies and definitions of subsequent IPD meta-analyses.
Improving estimates of antimalarial drug resistance to support decision-making
Efforts to support the translation of evidence into policy change are at the heart of MARC SE-Africa’s work. Evidence, however, can be hard to come by. Therapeutic data can be slow and costly to collect, not all types of data are collected everywhere, and there can be a significant delay between data collection and publication or sharing.
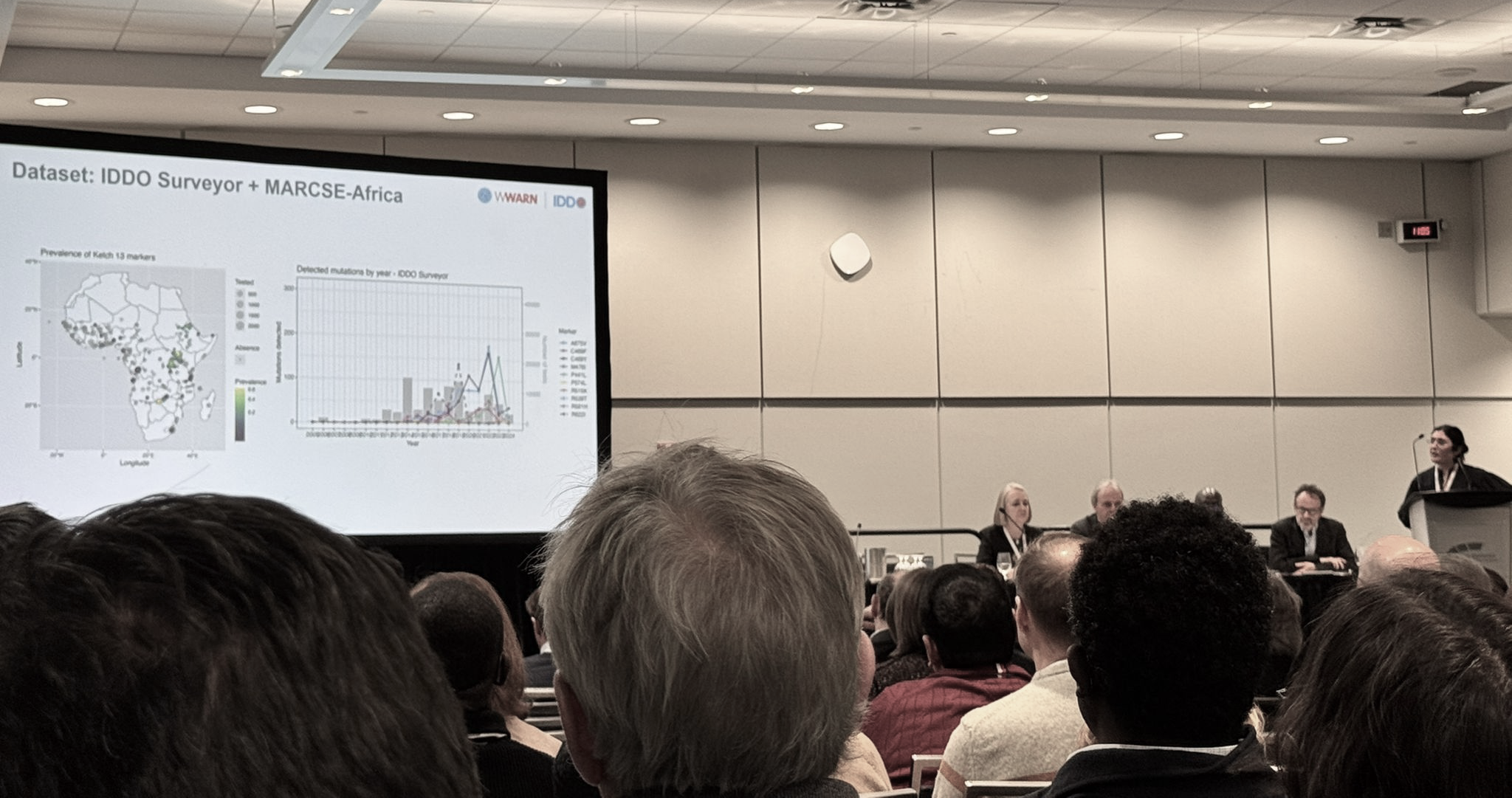
To address some of these barriers, the Worldwide Antimalarial Resistance Network (WWARN), a MARC SE-Africa project partner, is developing geospatial models: using available data to predict the changing prevalence of antimalarial drug resistance markers in Africa, including in regions where surveillance data are sparse. This work was presented by Dr Lucinda Harrison, who uses Gaussian Process modelling and Bayesian inference to create continental-scale maps of the prevalence of antimalarial drug resistance markers, as prevalence changes in space and time.
Dr Lucinda Harrison presenting at the ASTMH.
Her research so far has focused on K13 markers of artemisinin partial resistance, together with four mutations in the Pfcrt and Pfmdr1 genes associated with parasite resistance to the ACT partner drugs lumefantrine and amodiaquine, which are given in combination with artemisinin. By estimating parasite resistance to both of the drugs in an ACT, researchers hope to begin to understand the spatial and temporal distribution of ACT efficacy. These maps will be able to guide response efforts, including the adoption of multiple first-line therapies, where multiple ACTs are used in the same population, and triple artemisinin combination therapies, where two partner drugs are given to patients in combination with an artemisinin derivative.
The work presented at the ASTMH symposium highlights progress toward the consortium’s goal of providing quality, up-to-date information to support decision-making by national malaria control programme managers and other stakeholders. Antimalarial drug resistance in Africa is growing, the stakes are high, and innovative strategies are urgently needed. The MARC SE-Africa remains committed to supporting national and global stakeholders in developing the most suitable approaches to tackle this public health threat.
Explore this work further through MARC SE-Africa tools, interviews and webinars: